Equitable impact of an AI-driven breast cancer screening workflow in real-world US-wide deployment
This large real-world study demonstrated that a multistage AI-driven workflow for screening mammography deployed across several diverse US screening practices was associated with improved CDR across all prespecified breast density and race and ethnicity subpopulations. For the overall population, the CDR increased by 0.99 per 1,000 screens (4.59 to 5.58, P 1 also improved for the whole population and all powered subpopulations of interest in both the unadjusted and adjusted analysis. While RR increased by 5.7% (10.6 to 11.1, P = 0.015) overall, the increase in PPV1 suggests that additional recalls and diagnostic evaluations were appropriate because they led to a higher rate of additional cancer diagnoses. Increases in CDR held for women with dense and non-dense breasts, as well as for Black, non-Hispanic; Hispanic; and white, non-Hispanic women. Our results suggest that the multistage AI-driven workflow would not widen existing disparities in US screening outcomes, but rather could provide equitable benefits across key subpopulations of women. This level of increase in the CDR represents a potential additional 34,097 cancers found through early breast cancer screening over the 43 million mammograms performed in the USA each year, assuming that 80% of these are screening mammograms13.
The overall CDR increase observed here of 21.6% is greater than estimates of increased CDR (11%) associated with double reading 100% of exams in the USA18, highlighting the efficiency of combining a CADe/x device with a safeguard review in which only 8% of cases required a second review. This CDR increase is in addition to that already expected from a transition from full-field digital mammography to DBT of approximately 36% (ref. 19). Finally, the CDR increase was greater than that reported in ref. 5, which found an increase of 0.7 cancers per 1,000 screens, or ref. 9, which found a 17% increase in CDR in a double-reading standard-of-care cohort. The study in ref. 5 was of a prospective trial of 16,000 exams implementing an additional review process, analogous to the SafeGuard Review presented here, but in a European screening setting with double reading of full-field digital mammograms in women with 2-year screening intervals. References 9,10 demonstrated that, in the European double-reading setting, replacing one of the two readers with AI can achieve an increase in CDR or non-inferior CDR, respectively, alongside a decrease in the RR. However, double reading is not standard in the USA, so it is difficult to directly compare results in Europe with the USA. These different results highlight the importance of demonstrating the effectiveness of AI-assisted screening across varied populations and within the context of different workflows, screening paradigms and algorithm versions.
The CDR was 22.7% higher for women with dense breasts with versus without the multistage AI-driven workflow, suggesting that it may help address concerns for missed cancers in this subpopulation. With new US federal mandates requiring that women be informed of their density category after each screening mammogram20,21, the multistage AI-driven workflow may represent a welcome choice for women with dense breasts. These results are in contrast to those recently reported by ref. 6, which showed a non-significant improvement of CDR in dense breasts over a large age-restricted (50–69 years) prospective European cohort; however, this study used a different AI algorithm and different workflow where AI assistance was added to double reading.
Black and Hispanic women showed large relative improvements in their CDR (20.4% and 21.8%, respectively). Absolute increases in CDR were smaller for Black, non-Hispanic and Hispanic women than for white, non-Hispanic women, which can be explained by the lower reported incidence of cancer in Black, non-Hispanic and in Hispanic than in white, non-Hispanic women22,23 that is also seen in our data (Fig. 3). One of the driving forces for the recent revisions to the US Preventive Services Task Force screening recommendations for starting age of 40 years rather than 50 years was to improve health equity in breast cancer outcomes, especially for Black women24. By increasing the CDR, our study suggests that the multistage AI-driven workflow may facilitate the detection of cancers in earlier screening exams for racial and ethnic minorities, a population that has historically faced breast cancer diagnosis at later stages with worse morbidity and mortality24.
The clinically meaningful and statistically significant increase in PPV1 in the whole population and trend observed across all subpopulations of interest indicate that the additional recalls made with the multistage AI-driven workflow resulted in detecting additional cancers at a higher rate than the standard of care. Although the absolute increase in PPV1 was smaller for Black, non-Hispanic women than it was for white women (0.60 versus 0.95), the adjusted model did not demonstrate a statistically significant difference in the impact of the multistage AI-driven workflow on different racial and ethnic subpopulations. This suggests that, when demographic and radiologist-level factors are controlled, the relationship between the multistage AI-driven workflow and CDR, RR and PPV1 is similar for all subpopulations.
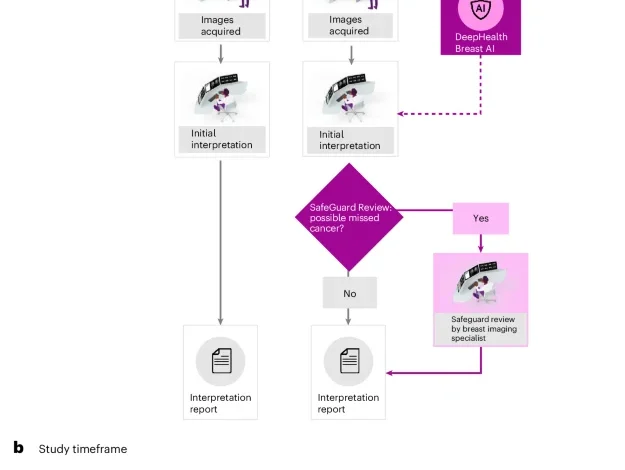
The strengths of our study include that this is one of the largest real-world US studies evaluating mammography screening with AI so far and includes data across 4 states, 109 individual sites and 96 individual radiologists. Most previous studies measuring CDR with DBT have been small and performed predominantly in academic research centres2,3. In contrast, our study represents real-world evidence collected from a large number of geographically diverse outpatient imaging centres and may better reflect the average US patient experience. The combination of (1) a CADe/x device on all cases and (2) a safeguard review by an expert reviewer for high-suspicion cases interpreted as normal by the initial radiologist is unique, particularly in a single-reading paradigm. The second-stage SafeGuard Review provides a process analogous to the consensus review in double-reading screening programmes in which all exams are read by at least two radiologists. However, in our workflow, only a small set of patients (8%) at highest risk for having cancer are double read. This enables nearly the full cancer detection benefits of double reading for 25. Finally, we observe similar changes in CDR, RR and PPV1 across the radiology practices (Supplementary Table 4) indicating that the AI algorithm and SafeGuard Review workflow are generalizable across the diverse set of practices investigated.
There are also several limitations to our study. First, there were insufficient follow-up data after screening to report sensitivity, specificity, false-negative rates, interval cancers or cancer stage at diagnosis. However, previous work comparing radiologist performance with versus without this CADe/x device (in both cases without the SafeGuard Review component) showed that radiologists improved sensitivity (80.8% without versus 89.6% with the device, P P = 0.65)14. In addition, the same study showed that radiologists reading with DeepHealth Breast AI had improved sensitivity across all lesion sizes and pathologies (invasive versus non-invasive), and ref. 26 reported similar distributions of invasive and triple negative cancers using the SafeGuard Review workflow described here compared with cancers identified without AI assistance. Second, it was not possible to extract the clinical impact of the CADe/x device from the SafeGuard Review owing to the unique aspects of the AI-driven workflow (for example, integration with existing imaging viewing software; workflow paths that include both the CADe/x and SafeGuard Review devices on a single exam; and user training and knowledge of both devices). Our results are therefore applicable to only the device under investigation. Third, we chose not to correct for multiple comparisons because our outcomes were highly correlated (for example, Black, non-Hispanic women were also included in the whole population; CDR, RR and PPV1 are related by radiologist behaviour and so on). However, we do account for correlation in the data through the adjusted generalized estimating equations models, and these adjusted results support the conclusions drawn from the unadjusted results. Finally, the cohorts were divided into two sequential groups in this real-world observational study, which does not control for unknown biases and confounders in the patient groups as a randomized trial would. However, the study prioritized external generalizability by assessing the AI workflow in a real-world clinical setting, thus avoiding biases that could arise from a highly controlled interventional study. Comparison between demographics, however, showed similar patient characteristics between groups, and these main confounders were controlled in the adjusted analysis.
In summary, the ASSURE study presents large-scale, real-world evidence that using a multistage AI-driven workflow is associated with improved mammography screening performance for the population as a whole and across density and key race and ethnicity subpopulations. These results demonstrate that the multistage AI-driven workflow can provide significant and equitable cancer detection benefits to women.





