Capricor says its cell therapy improved heart and muscle function in Duchenne patients
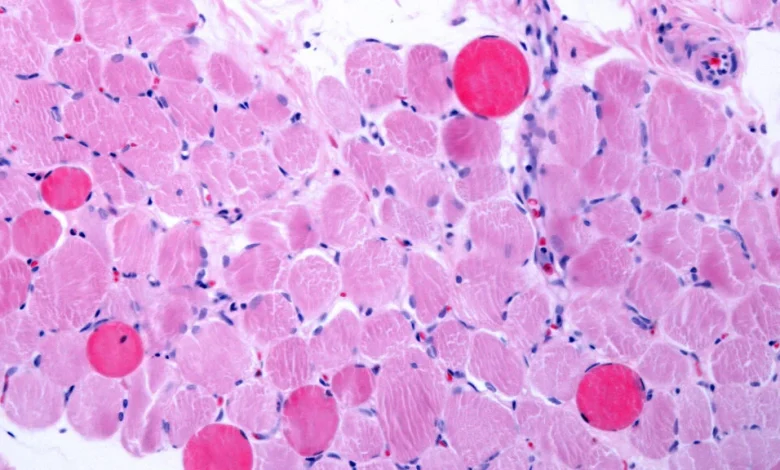
Capricor Therapeutics said Wednesday that its cell therapy significantly improved muscle and heart function in patients with Duchenne muscular dystrophy — dual outcomes that achieved the main goals of a Phase 3 study and will likely bolster the treatment’s future amidst an already contentious regulatory review.
In July, the Food and Drug Administration rejected the Capricor treatment, called deramiocel, telling the company its application, based on mixed results from a prior study, lacked “substantial evidence of effectiveness.” The decision was reportedly made by Vinay Prasad, the FDA’s top regulator of cell and gene therapies, overruling staffers who supported the therapy’s approval.
Capricor CEO Linda Marban said she thinks the new, positive results from a larger, placebo-controlled study are sufficiently persuasive to reverse the FDA’s prior decision.
STAT+ Exclusive Story
Already have an account? Log in
This article is exclusive to STAT+ subscribers
Unlock this article — plus daily coverage and analysis of the biotech sector — by subscribing to STAT+.
Already have an account? Log in
Individual plans
Group plans
View All Plans
To read the rest of this story subscribe to STAT+.
Subscribe





