Clascoterone 5% Delivers Strong Phase 3 Hair-Growth Results
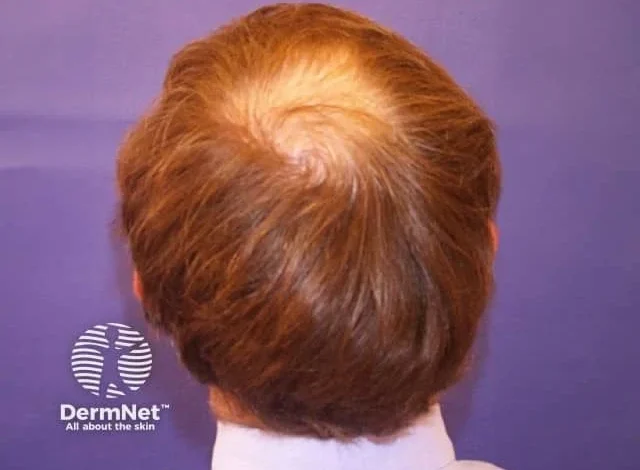
Cosmo Pharmaceuticals has reported encouraging topline findings from 2 large, identically designed phase 3 trials evaluating clascoterone 5% topical solution for male androgenetic alopecia (AGA). If ultimately approved, the drug could represent the first new therapeutic mechanism for AGA in over 30 years—a notable development for a condition with high prevalence and limited treatment options.1
Addressing an Old Problem With a New Mechanism
Male AGA affects an estimated 1.2–2 billion men worldwide and is characterized by progressive miniaturization of hair follicles driven by dihydrotestosterone (DHT). Existing treatments, including oral 5-α reductase inhibitors and topical minoxidil, have demonstrated benefit but are associated with either systemic exposure, variable efficacy, or issues with long-term adherence.2
Clascoterone 5% topical solution employs a different approach: local androgen receptor inhibition at the follicular level, designed to reduce DHT signaling without meaningful systemic absorption. This mechanism may appeal to patients who avoid oral treatments due to concerns about hormonal effects or other systemic adverse events.
Study Design and Population
The 2 phase 3 trials—the SCALP 1 (NCT05910450) and SCALP 2 studies (NCT05914805)—were multicenter, randomized, double-blind, vehicle-controlled trials conducted across the United States and Europe. A total of 1,465 men aged over 18 were enrolled. Each study included a 6-month double-blind treatment phase followed by a 6-month single-blind extension.
Co-primary endpoints were:
- Target Area Hair Count (TAHC)
- Patient-Reported Outcomes (PROs) assessing perceived improvement in hair growth
Efficacy Results
Both trials demonstrated statistically significant improvements in hair growth compared with vehicle. In one study, clascoterone produced a 5.39-fold (539%) relative improvement in TAHC, while the second trial showed a 1.68-fold (168%) relative improvement. The magnitude of difference between the 2 studies is not unusual for hair-growth research, which often shows variability based on baseline disease severity, regional demographics, or site-level differences in assessment technique.
Importantly, PRO data reinforced the objective findings. One study met its PRO endpoint while the other demonstrated a positive trend; combined analysis across both trials achieved statistical significance. Alignment between patient perception and investigator-assessed improvement is clinically relevant, particularly in a condition where self-image and treatment satisfaction play a central role in adherence.
Safety and Tolerability
Treatment-emergent adverse events (TEAEs) were similar between active treatment and vehicle groups. Most TEAEs were not considered drug-related. The absence of meaningful systemic androgen blockade—supported by the known safety profile of clascoterone in its approved acne formulation—may differentiate this treatment from oral options.
Maria Hordinsky, MD, professor of dermatology at the University of Minnesota, emphasized the clinical relevance of these findings. “For decades, patients have had to choose between available treatment options with limited efficacy or safety issues due to systemic hormonal exposure,” she noted. “These findings show the potential for clascoterone 5% topical solution to change that equation by delivering real, measurable regrowth with negligible systemic exposure.”
Implications for Clinical Practice
If ultimately approved by regulatory agencies, clascoterone 5% solution could expand treatment choices for AGA by providing a mechanistically distinct option that targets the androgen receptor directly in the follicle. This approach may be particularly attractive for patients unwilling to use oral 5-α reductase inhibitors or those who have not responded to current therapies.
Cosmo Pharmaceuticals’ CEO Giovanni Di Napoli underscored the potential impact, stating: “For the first time in more than thirty years, we have a completely new mechanism with the potential to truly change that reality.” While enthusiasm must be tempered until full datasets and regulatory decisions are available, the early data suggest the therapy may meaningfully address a longstanding gap in the dermatologic armamentarium.
Next Steps
Cosmo plans to complete the required 12-month safety follow-up by spring 2026, after which parallel FDA and EMA submissions are expected. If approved, clascoterone 5% solution would become the first topical androgen receptor inhibitor indicated for AGA.
Clinicians should watch for forthcoming detailed analyses, including subgroup evaluations, long-term safety data, and guidance on use alongside existing therapies—areas that will help contextualize how this product might be integrated into routine practice.
References
- Cosmo announces breakthrough pphase III topline results from Scalp 1 and Scalp 2 for clascoterone 5% solution in male hair loss, showing up to 539% relative improvement in target-area hair count vs placebo; US and EU submissions are underway. News release. Cosmo Pharmaceuticals. Published December 3, 2025. Accessed December 4, 2025. https://www.cosmopharma.com/news/cosmo-announces-breakthrough-phase-iii-topline-results-from-scalp-1-and-scalp-2-for-clascoterone-5-solution-in-male-hair-loss-showing-up-to-539-relative-improvement-in-target-area-hair-count-vs-place
- Chen S, Li L, Ding W, Zhu Y, Zhou N. Androgenetic alopecia: An update on pathogenesis and pharmacological treatment. Drug Des Devel Ther. 2025 Aug 25;19:7349-7363. doi: 10.2147/DDDT.S542000.




